
Dr. K. L. Mittal, Dr. Robert H. Lacombe
Origin of Surface Energy
In the December 2013 issue of this blog we noted that nearly all the information we commonly acquire about any given surface comes from the radiation reflected from it. We also noted that of the entire range of the electromagnetic spectrum available that a surface could possibly radiate, we detect only the so called visible spectrum which amounts to barely 2% of what could be emitted. Thus what our eyes alone tell us about what is happening on a given surface is very limited indeed. Not only that but the type of information is limited to basically the bulk geometry, gross surface morphology and color.
The fact of the matter is that an entire universe of important physical properties remain invisible to our eyes. The invisible property we want to explore here in fact cannot be seen even in principle. This property is what is called the surface energy and to get a picture of it we need the apparatus of thermodynamics.
The whole concept of energy is rather subtle and intricate in general. In particular it can take many forms including:
- Electromagnetic energy stored in electric fields
- Magnetic energy stored in magnetic fields
- Thermal energy stored in any material at a finite temperature
- Potential energy of any mass in a gravitational field
- Kinetic energy of any moving object
- Relativistic energy of any massive object as given by Einstein’s famous formula E = mc2
- etc.
However, for our purposes we only need to understand the elastic energy stored in common solids and we can approach this by considering the behavior of a common spring. Stretch or compress a spring and it will store a certain amount of elastic energy which can be perceived by allowing the spring to return to its equilibrium length. Much the same type of behavior goes on in common solids. Figure (1) gives a highly idealized but reasonably realistic picture of a solid material viewed at the atomic level.
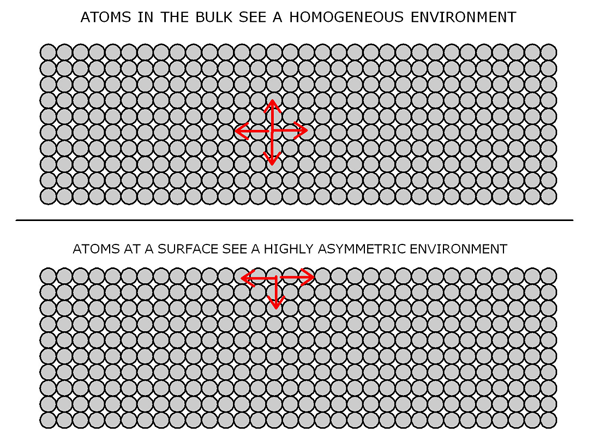
Fig. 1: Schematic diagram of a solid viewed at the atomic level. To a first approximation the atoms can be thought of as being held together by microscopic springs which account for the elastic properties of the material.
The atoms/molecules in a given solid are held together by atomic and intermolecular forces which arise from the rather complex electromagnetic interactions among the electrons and nuclei which make up the bulk of any material. Fortunately, near equilibrium and for small deformations these interactions behave in a linear fashion very much like the behavior of simple springs. Thus as Robert Hook pointed out more than a century ago the restoring force tending to bind the atoms together increases in a linear fashion as they tend to separate from one another. Things get quite a bit more complicated at large deformations but that need not concern us here.
Referring to the upper diagram in figure (1) we see that a typical atom in the bulk of our hypothetical solid feels either tensile or compressive loads from all directions and much the same is experienced by all the rest of the atoms in the deep interior of the solid. However, the situation is quite a bit different for those atoms at or near the surface as shown in the bottom diagram of figure (1). Looking down into the bulk of the material they see that same forces as the bulk atoms do but now there is no material on top which creates a highly asymmetrical situation. It is precisely this asymmetry that gives rise to the unique surface tension or surface energy of the solid.
A Word on Units
Perhaps one of the most confusing things about surface energies are the units they are expressed in so we take a quick break here to clear up this issue. Going back to our spring, if we stretch it there arises an immediate force tending to return it to the un stretched length. Current international convention expresses this force in the standard SI units[1] of newtons. All systems of units are essentially arbitrary but it is nonetheless important to settle on a common standard. Thus the common SI units for force are the newton with the dyne and the pound also in use but not considered standard by the international community. The newton is the canonical unit of the international scientific community and the dyne is a scaled down derivative. The pound is an archaic holdover from the past but is still much in use in commercial transactions especially in the USA.
With the concept of force now rigorously defined we move on to the concept of energy again using our standard apple as a prop. Force times distance is energy in the form or work. Let’s assume that the apple weighs exactly 1 newton. If we raise the apple from the ground to a height of 1 meter we will have done 1 joules worth of work or putting it a little differently we will have increased the apple’s gravitational energy by one joule.
Getting back to our spring, it stores what is called elastic energy. As stated above energy is force times distance and the restoring force of a spring is proportional to the extension so the energy stored in an extended spring is proportional to force times distance squared. These ideas can all be compactly summarized in the following formulas:
F = -k x (Restoring force exerted by a stretched spring) (1)
Where:
F = Force in newtons
x = Displacement in meters
k = Spring constant in newtons/meter
The minus sign in Eq(1) indicates the force is always a restoring force tending to oppose any extension or compression.
The energy stored in the spring is the integral of Eq(1) from 0 to some extension d:
W = IFxdx = -(½)kd2 (Energy stored in stretched spring) (2)
Thus if our spring has a spring constant of 1 newton/meter and we extend it to a distance of 1 meter it will pull back with a force of 1 newton. Also it will store an energy of ½ joule.
We can think of the spring as having a tension of 1 newton/meter which is just another name for the spring constant. Turning now to the physical surface of a polymer such as nylon the springs binding the atomic units together would have a tension of about 8×10-25 newtons/meter and this is the basis of the so called surface tension or surface energy of this material. Now in practice we do not deal with such impossibly small numbers so we need to scale things up a bit. In the case of our nylon, one square meter of the surface will contain something like 5×1022 molecular bonds among the surface moieties or in terms of our simple spring model 5×1022 springs. So we take the surface tension of our polymer to be the tension in a single bond times the total number of bonds in a square meter which for our nylon material comes to 40×10-3 newtons/meter. This is still to awkward so we introduce the milli newton (abbr mN) which is 10-3 newtons so nylon now has a surface tension of 40mN/m (abbreviating the meter as m). Well why stop here. We can do a little algebra on the units and say that 40mN/m is the same as 40mN-m/m2 by multiplying numerator and denominator by m. Now the mN-m we recognize as a mJ or milli joule and so our nylon can be thought of as having a surface tension (aka surface energy) of 40mJ/m2. And it does not stop here. Many folks dealing with surface tension measurements would rather not have to deal with the milli prefix and such huge surface areas as a square meter. It is more convenient to scale down the force unit to dynes (10-5 newtons) and use square centimeters (abbr cm) instead of square meters. Thus 40mN/m scales down to 40 dynes/cm.
Real Solid Surfaces
Now that we have pinned down suitable units for measuring surface energies we can have a closer look at real material surfaces. The diagrams in figure 1 give a much better depiction of a liquid surface than they do for a solid. Liquids have a high mobility and can always adjust their configuration to give a uniform surface of minimum surface tension. Not so with solids. The surface configuration of a solid depends sensitively on the thermal-mechanical loading conditions under which it was created. Was the material cooled rapidly or slowly? What type of loads if any were active during the cooling process?
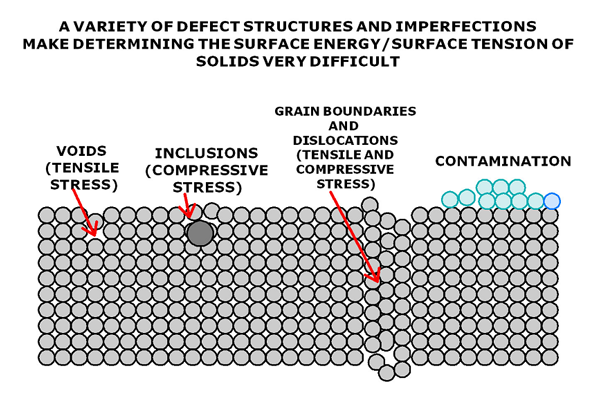
Figure 2 gives a more realistic depiction of what a typical solid surface looks like. This figure depicts 4 typical surface flaws that can significantly alter the surface energy of any real solid:
- VOIDS: Materials that have been rapidly quenched may not have time to completely condense giving rise to voids which are a source of tensile stress that will alter the surface energy in their vicinity.
- INCLUSIONS: No material is 100% pure and contamination species have a strong tendency to migrate toward surfaces where they upset the normal packing and in many cases give rise to a local compressive stress.
- GRAIN BOUNDARIES/DISLOCATIONS: Nearly all crystalline and semi crystalline materials are polycrystalline in nature. That is they are made up of an aggregate of a large number of small crystals all packed together in no particular order. The boundary where two crystallites meet form what is called a grain boundary. Further the misalignment of planes within the crystalline give rise to what are called dislocations. These and other imperfections can give rise to local stress fields where they intersect a surface that again alter the local surface energy.
- CONTAMINATION: Of all the surface imperfections contamination layers have the profoundest effect on surface energies. Real material objects sit around on benches in the lab or other platforms where they are subject to constant bombardment from all the contaminants and gases in a typical atmosphere not the mention the greasy fingers of human handlers.
Needless to say all of these considerations make the accurate measurement of the surface energies of solids a rather tricky business. But enough for now. We take up this question in the next chapter.
The author invites any inquiries or comments.
[1]The International System of Units (abbreviated SI from French: Le Système international d’unités). A bunch of folks got together and formed the General Conference on Weights and Measures, an organization set up by the Convention of the Metre in 1875, which succeeded in bringing together many international organizations to agree not only the definitions of the SI, but also rules on writing and presenting measurements in a standardized manner around the globe.
Recommend






Write a comment