
Dr. K. L. Mittal, Dr. Robert H. Lacombe
We continue the INVISIBLE UNIVERSE blog after a delay due to our heavy involvement in the recently completed 9th INTERNATIONAL SYMPOSIUM ON CONTACT ANGLE, WETTABILITY AND ADHESION held at Lehigh University June 16-18, 2014.
Speaking of CONTACT ANGLE phenomena this would be a good time to review this topic as the contact angle method is by far and away the most popular surface analysis method in use today. This technique is of special interest to anyone doing surface modification either by plasma or any other method as it is the simplest and least expensive method for analyzing the impact of any surface treatment whatever. It is fortunate that our office has received a recently published volume on this topic which we will review shortly but first a rudimentary introduction to the concept of contact angle behavior of droplets for the sake of those who may be new to this topic.
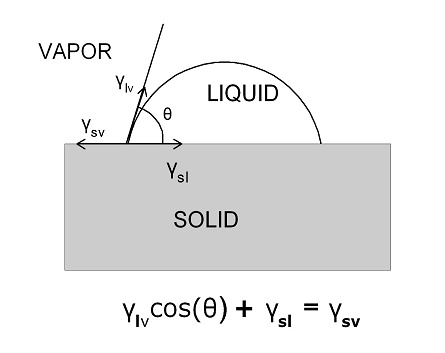
Fig.(1). Classic definition of the equilibrium contact angle of a drop of liquid on a surface as the balance of three surface tensions.
When a drop of some liquid is placed on a surface it will typically bead up and form a sessile drop as exhibited in Fig.(1). The angle that the edge of the drop makes with the underlying solid is determined by the balance of three surface tensions or surface energies if you prefer. The concept of surface energy has been covered in some detail in the February 2014 issue of this blog so it will not be reviewed here only to point out that surface tensions as measured in Nt/m are dimensionally the same as surface energies (J/m2) which are readily derived from surface tensions by multiplying the numerator and denominator of the units by m (meter) giving Nt-m/m2 or J(joules)/m2.
And now to our book review:
Wetting of Real Surfaces, By Edward Yu. Bormashenko, (Walter de Gruyter GmbH, Berlin/Boston, 2013)
In this rather compact monograph Prof. Bormashenko provides a rather comprehensive account of the theoretical developments in the field of contact angle and wettability of surfaces.
Interestingly, Prof. Bormashenko points out that the field of contact angle and wettability remained rather a backwater endeavor in the field of modern physics from the time of Thomas Young’s pioneering work up to roughly the 1990’s despite the fact that scientific heavyweights such as Einstein, Schrödinger and Bohr devoted a significant portion of their research activity to this topic. Much of this stems from the fact that surfaces presented a rather messy and intractable research topic due to the difficulty in obtaining well defined surfaces free of contamination and other defects. Indeed the eminent theoretical physicist Wolfgang Pauli remarked that “God created matter but the Devil created surfaces”. Thus the solid state physics literature up to about the early 1980’s tended to be dominated by topics such as superconductivity, electronic band structure, phase transitions, semiconductors and similar topics dealing primarily with the bulk behavior of solids.
This all started to change significantly by about the 1980’s being led in large part by the microelectronics industry which was fabricating multilevel thin film structures which were becoming more and more dominated by interfacial surfaces between metals, insulators and semiconductors. Even by the early 1970’s it was becoming apparent that in order to fabricate devices with higher and higher circuit densities it was critical to understand the nature of the interactions between the various material components at their contact surfaces. This need was supported by advances in microscopy starting with electron microscopy and evolving further to electron tunneling microscopy and finally to the now ubiquitous atomic force microscopy. On top of this a number of surface analysis techniques emerged nearly too numerous to mention the most popular of which being X-ray Photoelectron Spectroscopy (XPS also called ESCA Electron Spectroscopy for Chemical Analysis).
The need for understanding surface properties was of course not limited to the microelectronics industry. The entire coatings industry needed to understand the wetting properties of various paints and inks and the biotechnology industry dealing with medical implants needed to understand how the surfaces of their devices would interact in the in vivo environment. The contact angle technique thus started to emerge as a low cost and highly sensitive method for exploring the wetting behavior of surfaces.
A critical juncture of sorts was achieved with the work of Barthlott and Neinhuis in 1997[1] who first studied the extreme hydrophobicity of the lotus leaf and its effect in removing all manner detritus from the leaf’s surface. This work lead to a literal explosion of work on the superhydrophobic effect and a variety of applications to self cleaning surfaces and other highly innovative technologies.
Getting back to Prof. Bormashenko’s volume a brief look at the table of contents reveals a rather wide range of topics:
- What is surface tension
- Wetting of ideal surfaces
- Contact angle hysteresis
- Dynamics of wetting
- Wetting of rough and chemically heterogeneous surfaces: the Wenzel and Cassie models
- Superhydrophobicity, superhydrophilicity and the rose petal effect
- Wetting transitions on rough surfaces
- Electrowetting and wetting in the presence of external fields
- Nonstick droplets
There is clearly not enough space here to cover all of the above topics in any detail so we will focus on chapter 6 dealing with super hydrophobic and hydrophilic phenomena which, as Prof. Bormashenko points out, are among the currently most actively researched topics in the contact angle field.
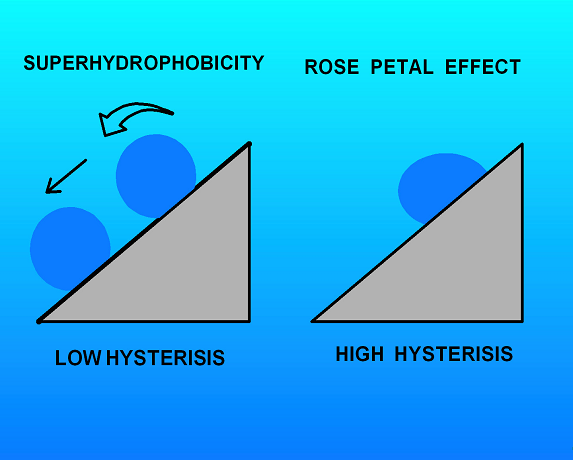
Fig.(2). Schematic illustrating the difference between a truly superhydrophobic surface and one exhibiting only a high contact angle
Interestingly, the author points out that exhibiting a high contact angle is not sufficient to define a state of superhydrophobicity as might casually be assumed. In addition the contacting water drop must also exhibit low contact angle hysterisis. That is the advancing and receding contact angles must be approximately the same. This property is required for the so called “lotus leaf” effect where water drops not only form with a high contact angle but also roll very easily off the leaf carrying any collected debris with them as shown in Fig.(2).
The counter example is the “rose petal” effect reported on by Jiang and co-workers.[2] These investigators looked at water droplets on rose petals which also form very high contact angles but unlike the lotus leaf case these drops also exhibit a very strong hysterisis. An immediate consequence is that these drops do not roll even when held at a steep angle as also shown in Fig.(2).
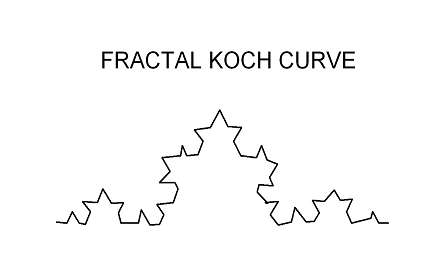
A further subtle point brought out in the chapter is the fact that the substrate material does not have to be highly hydrophobic in order to exhibit the superhydrophobic effect. The lotus leaf material is in fact hydrophilic. What gives rise to the superhydrophobic behavior is the hierarchical relief morphology of the surface. An example of such a structure would be the fractal Koch curve shown schematically in Fig.(3). The author goes on to analyze the wetting of these highly variegated surfaces in terms of the Wenzel and Cassie models covered in chapter 5.
In all this volume can be highly recommended to anyone interested in coming up to date on the latest theoretical developments in the rapidly expanding field of contact angle phenomena.
The author invites any inquiries or comments on this article.






Write a comment