
Khoren Sahagian
Micro fluidic devices and sensor surfaces may require a specific binding property or a capacity to react with a fluid. Functional groups such as amine, hydroxyl and carboxyl (to name a few) will evoke system specific responses such as binding to proteins, nucleotides, and adhesives. Plasma begins by removing organic surface contaminants by reducing them to volatile compounds. The nascent surface is subsequently reacted to process specific plasma chemistry. The intensity and duration of a plasma process is deterministic in the surface functionality and density that result. If the density of a functional moiety is either too high or too low this may hinder an intended surface reactivity.
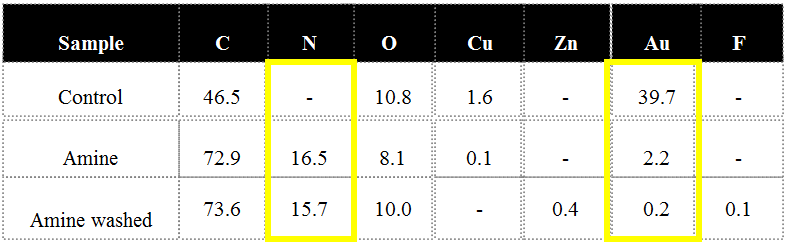
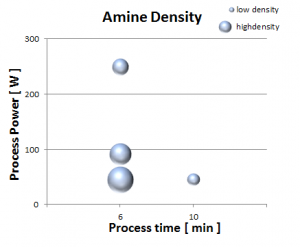
The table above illustrates the percentage of elemental nitrogen detected on a gold surface as measured by XPS before and after plasma modification. This amine is covalently bound to the surface meaning it is permanently incorporated onto the surface. This is exemplified by the persisting nitrogen composition post solvent wash (see ‘Amine washed’ in the table above). Different amine densities result from varying the plasma intensity and exposure. It is noteworthy that plasma processing isn’t a linear phenomenon and therefore judicious selection of power, pressure, and time may be necessary. More power and more time does not always translate to denser species loading. This is because a plasma operates between regimes that may be either addition or ablation dominant.
Recommend






Write a comment