
Dr. K. L. Mittal, Dr. Robert H. Lacombe
This issue of the SURFACE SCIENCE CORNER blog inaugurates a series of essays on the above mentioned topic concerning how we recognize the ubiquitous surfaces we look at all of our waking moments. The answer of course is through the subtle apparatus of our visual neurology which is activated by the light coming at us from all directions. The question then becomes what information is all this light carrying to our visual cortex. However, before we try to unravel this question we need to examine in a little more detail the nature of the light that is being reflected into our eyes from all directions.
The light coming at us is part of what is called the electromagnetic radiation spectrum the entire extent of which is displayed in figure (1). The striking thing about this figure is the enormous range of the spectrum covering some 20 orders of magnitude on the logarithmic scale shown in the figure. To print the entire figure on a linear scale would require a sheet of paper extending out to the edge of the solar system assuming 0.1 mm of sheet per 10Hz of frequency. The second remarkable feature of this diagram for our purposes is the fact that the range of frequencies of visible radiation which is what our eyes detect is less than 2% of the range. Though we detect a limited number of mechanical and thermal properties of surfaces through direct touch, the preponderance of our awareness of surfaces comes from reflected radiation. Going by the figure we see that our eyes are missing some 98% of what is potentially being reflected at us.
So what are we missing in particular. Lets consider first the infrared region from roughly 1011 to 1014 Hz. This is radiation contributed by the incessant atomic and molecular motions of the atoms and molecules which make up all matter. To our eyes all surfaces lying at rest are perfectly still certainly at the macroscopic level. However, consider for example a carbon atom associated with a particular bit of organic contamination residing on some apparently undisturbed surface at room temperature. An elementary thermodynamic calculation indicates that far from sitting still such an atom is vibrating in place at an average velocity near 300 m/sec. This motion combined with the motion of all the other atoms and molecules on the surface gives rise to infrared radiation which is beamed in all directions and is entirely invisible to our eyes. We can, however, detect the molecular vibrations of organic molecules on surfaces with the aid of specialized infrared spectrometers so we know they are there even though we cannot see them.
What else are we missing? Going to higher frequencies in the range of 1016 to 1019 Hz we find ourselves in the land of the X-rays which reflect information on atomic and molecular structure on the scale of a few angstroms or roughly 10-10 cm. If we could detect this radiation we would be able to see the atomic and molecular packing of all the species at the surface. Things like crystal structure, grain boundaries, dislocations and assorted other types of contamination and defects lying on the surface. In addition we would be able to detect the inherent roughness of the surface at the atomic and molecular scale. What to the eye would appear to be a perfectly smooth surface when observed with X-rays would appear to be quite rough an rugose. Such variable surface topography can have quite significant effects on common properties such as surface wettability.
Going to yet higher frequencies we find ourselves in the range of the gamma rays which live in the range from roughly 1019 to 1020 Hz. The gamma rays allow us to peer into the goings on in the atomic nucleus some 1000 times smaller than the typical atom. In particular, some nuclei are unstable and can disintegrate into smaller nuclei giving off gamma rays and other particles in the process. Most common materials are not radioactive but some do have contamination level concentrations of radioactive species which can give off barely detectable amounts of radiation. Now one might not expect that sub detectable levels of radiation would be of much concern to the practical product engineer manufacturing some wholly macroscopic device for industry. However, the world of surfaces can be quite subtle and engineers in the microelectronics industry got an elementary lesson in nuclear physics from that most common of common materials lead. It turns out that lead can harbor contamination levels of radioactive species the activity of which are quite invisible to our eyes as explained above. Lead has a long history of being used to make electrical connections in the electronics industry going back to at least the mid 19th century. It so happened that in the early 1980’s lead solder was being used to connect sensitive memory chips to ceramic substrates. The memory chips were of a special kind which utilized the very high resistivity of single crystal silicon to trap a small amount of charge in a small cell which formed the basis of an elementary unit of memory. A cell containing charge served as a boolean “1″ and an empty cell represented a boolean “0″. All well and good but all the cells had to be connected to the remaining computer circuitry using metal lines and interconnects and of course that old work horse lead served as one of the interconnect materials. The reader can now well guess what happened. The radioactive contaminant species in the lead would decay from time to time giving off not only a gamma ray but also a highly charged alpha particle. The alpha particle was the main mischief maker. Carrying a charge of +2 it does not travel very far in ordinary matter but where it does go it leaves behind a trail of ionization which can momentarily turn a highly resistive material like single crystal silicon into a good conductor along the path of the alpha particle. One can easily imagine a wayward alpha particle crashing into one of the silicon memory cells causing a charged cell to discharge along the ionization path left by the alpha. A memory register of the computer has now been randomly and irreversibly changed which is not good from the point of view of programming logic. If the affected register happened to contain an important logic instruction the result would easily lead to a serious programming error or simply machine lockup. The field engineers came to know this type of problem as a “soft” error since the ionization trail left by the alpha particle would quickly dissipate leaving the affected memory cell quite unharmed. Thus any attempt to locate the source of the error would be futile since no permanent hardware malfunction was involved. Such so called “soft” errors are the worst kind from the point of view of the field engineer. They come at random from seemingly nowhere and the culprit escapes without a trace. How this problem was eventually solved is a story for another time but for now it simply illustrates that what we cannot see coming off a surface can indeed bite us in wholly unsuspected ways.
If we now go to the lower frequencies from 104 to 1011 Hz we come across the radio and microwave bands. If we could detect these frequencies we would be able to see the myriad electronic and polarization currents which endlessly flow in all materials and give rise to a number of phenomena which affect us in various ways even though we cannot visually see them.
All this will be covered in future issues of the SURFACE SCIENCE CORNER blog.
The author invites any inquiries or comments.
Recommend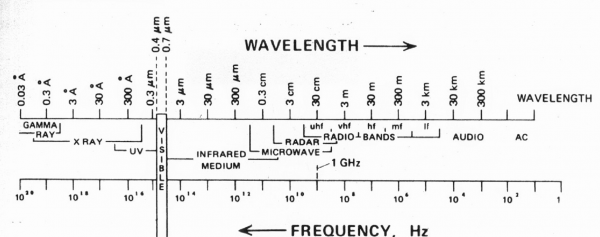






Write a comment